progress
November 2014
During Y1 significant goals have been achieved in WP 3 Reproduction and genetics - greater amberjack A large stock (~140 individuals with a body mass around 5-9 kg) of wild-caught greater amberjack was acquired by HCMR, ARGO, ITTICAL and distributed in cages (two stocks, HCMR and ARGO) as well as in tanks (three stocks, HCMR, ARGO, ITTICAL). Although the ITTICAL stock was lost for the occurrence of an infestation of the parasite A. ocellatum, preliminary spawning induction experiments were executed in the remaining stocks (Task 3.2, led by HCMR) and important data were obtained for Y2 hormone induction experiments. Moreover, an ad hoc designed egg collector allowed the collection of high quality fertilized eggs from greater amberjack maintained in sea cages (Task 3.5, led by HCMR). A large number of eggs was obtained with both natural and hormone-induced spawnings from greater amberjack reared in tanks (Task 3.3, led by FCPCT). In greater amberjack individuals born in captivity (F1 generation), neither spontaneous nor hormone induced spawns provided fertilized eggs (Task 3.4, led by IEO), underlining the necessity to improve hormonal treatment as well as fish nutritional and health status. Biological samples were collected from wild greater amberjack caught in the waters around Lampedusa (Sicily, Italy) and will be delivered to the relevant Partners (Task 3.1, led by UNIBA). Preliminary information on wild greater amberjack reproductive cycle were obtained by histological evaluation of ovaries and testes. Vitellogenin A mRNA was amplified through PCR carried out using primer pairs designed against conserved sequences from other Perciform species.
During Y1 significant goals have been achieved in WP 3 Reproduction and genetics - greater amberjack A large stock (~140 individuals with a body mass around 5-9 kg) of wild-caught greater amberjack was acquired by HCMR, ARGO, ITTICAL and distributed in cages (two stocks, HCMR and ARGO) as well as in tanks (three stocks, HCMR, ARGO, ITTICAL). Although the ITTICAL stock was lost for the occurrence of an infestation of the parasite A. ocellatum, preliminary spawning induction experiments were executed in the remaining stocks (Task 3.2, led by HCMR) and important data were obtained for Y2 hormone induction experiments. Moreover, an ad hoc designed egg collector allowed the collection of high quality fertilized eggs from greater amberjack maintained in sea cages (Task 3.5, led by HCMR). A large number of eggs was obtained with both natural and hormone-induced spawnings from greater amberjack reared in tanks (Task 3.3, led by FCPCT). In greater amberjack individuals born in captivity (F1 generation), neither spontaneous nor hormone induced spawns provided fertilized eggs (Task 3.4, led by IEO), underlining the necessity to improve hormonal treatment as well as fish nutritional and health status. Biological samples were collected from wild greater amberjack caught in the waters around Lampedusa (Sicily, Italy) and will be delivered to the relevant Partners (Task 3.1, led by UNIBA). Preliminary information on wild greater amberjack reproductive cycle were obtained by histological evaluation of ovaries and testes. Vitellogenin A mRNA was amplified through PCR carried out using primer pairs designed against conserved sequences from other Perciform species.
|
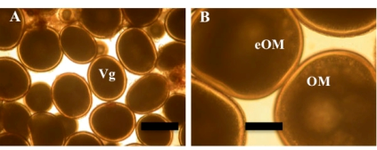
Figure 1: Wet mount photographs of greater amberjack oocytes obtained from the HCMR broodstocks maintained in sea cage. A, oocytes in full vitellogenesis (Vg). B, oocyte in maturation. Bar = 200 μm
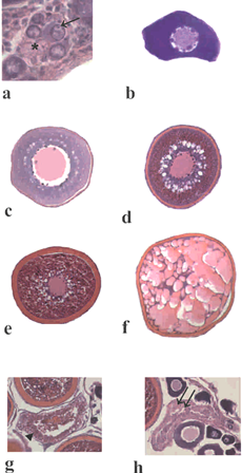
Figure 2: Greater amberjack cells in different developmental stages. a) Oogonia (aterisk) and chromatin-nucleolus stage oocyte (arrow). b) Perinucleolar stage oocyte. c) Cortical alveoli stage oocyte. d) Early vitellogenic oocyte. e) Late vitellogenic oocyte. f) Hydrated oocyte. g) Atretic vitellogenic follicle (arrowhead). h) Post-ovulatory follicle (double arrows).
|
Participating organizations (WP leader in bold): P1. HCMR, P2. FCPCT, P4. IOLR, P8. IEO, P13. UNIBA, P14. IFREMER, P15. ULL, P23. ARGO and P24. ITTICAL
Task 3.1 Description of the reproductive cycle of greater amberjack (led by UNIBA). Wild-caught broodstocks will be maintained in captivity by one of the SMEs. Broodstock will be sacrificed at three different times during the reproductive season. Blood, brains, pituitaries, gonads, muscle and liver will be sampled by scientist from different Partners to study the reproductive cycle, and spines will be collected for age determination. Wild fish will be sampled at the same time as a reference for evaluating reproductive function of captive fish. Proliferation and/or apoptosis of germ cells during spermatogenesis will be examined using Proliferating Cell Nuclear Antigen (PCNA) and the TUNEL method. Characterization of amberjack sperm will be done using CASA, looking at % spermatozoa motility, velocity, linearity of tracks, sperm membrane integrity by eosin/nigrosin, sperm viability and sperm energetic status using ATP measurement. A comparison of liver vitellogenin (Vg) and ovary Vg receptor (VgR) gene expression between captive and wild females will be assessed by cDNA sequencing and real time-PCR (qPCR); an analysis of oocyte yolk accumulation will be performed on histological sections using image analysis. Measurement of the sex steroid hormones T, E2, 11-KT and 17,20β DHP will be done using ELISAs and the expression of LH and FSH, as well as their plasma protein levels will be measured. Recombinant LH and FSH proteins will be produced and used to develop the respective hormone-specific ELISAs. Similar methodology will be applied to develop an ELISA for measuring leptin, a key metabolic hormone known to interact with the endocrine system to provide critical information about the nutritional status. The nutritional status of the captive and wild fish will be compared. The muscle, liver, and gonads samples will be analyzed to determine: (a) proximate and fatty acid composition, including specific lipid classes (i.e. phosphatidyl serine, inositol, choline and ethanolamine), (b) vitamin C and E and (c) carotenoids.
Task 3.2 Development of an optimized spawning induction protocol for captive greater amberjack in the Mediterranean (led by HCMR). Mature wild fish will be captured using a purse seine by two of the SMEs. Using these fish, captive broodstocks (n=10 males and females) will be established in HCMR, ARGO and ITICAL and will be maintained in tanks and cages. After 1 year in captivity (Y2), two different methods will be examined, either multiple GnRHa injections given every 7 days or implants for sustained release of GnRHa spanning 4-weeks. In Y3, two different doses of the most effective treatment from Y2 will be evaluated. In Y4, the timing of application (late June/early July) of the most effective treatment/dose will be optimized, based on the data obtained from the previous years. In the SME Partners, spawning induction on a single broodstock will be undertaken to validate protocols and to ensure egg production for the WP4 Larval husbandry. Evaluation of spawning quality will be carried out for all spawns, using individual egg incubations in 96-well microtiter plates.
Task 3.3 Development of an optimized spawning induction protocol for captive greater amberjack in the eastern Atlantic (led by FCPCT). Mature wild-caught fish adapted to culture conditions will be divided into 3 groups of 6 males and 6 females. At the onset of the spawning season in the eastern Atlantic (April-May), after evaluation of reproductive status fish will be treated with either single GnRHa injections every 7-10 days or GnRHa implants (that will be produced by HCMR) every 3 weeks, and one group will be left untreated as control. Evaluation of the spawning quality will be carried out for all spawns.
Task 3.4 Development of an optimized spawning induction protocol for F1 greater amberjack in the eastern Atlantic (led by IEO). Broodstock will be divided into 4 groups of 8 males and 8 females. At the beginning of the spawning season the reproductive status of fish will be evaluated (same procedures as 2.1.3; Mylonas et al., 2004) and fish treated with GnRHa implants. Females will be given GnRHa implants of different doses (0, 25, 50, 75 µg kg-1) while males will be implanted with 30 µg kg-1. The evaluation of the spawning quality will be carried out for all spawns (same procedure as 2.1.2; Mylonas et al., 2004). GnRHa implant treatments will be repeated when spawning stops. At the time of each GnRHa implantation and the end of the spawning season the following parameters will be determined: (a) plasma levels of the sex steroid hormones (HCMR); (b) plasma levels of triglycerides, cholesterol, protein and enzymes (GPT, GOT, alcaline phosphatase, cholinesterase and amylase); (c) cortisol, glucose and lactate; and (d) electrolytes, in order to determine hematological and biochemical indicators of health and welfare.
Task 3.5 Spawning induction of greater amberjack and egg collection in cages (led by HCMR). Cage spawning is expected to facilitate the management of greater amberjack broodstocks that may exceed 30 kg in commercial operations. Therefore, DIVERSIFY will employ methods used in a previous EU 7th FP project (SELFDOTT) for the collection of fertilized eggs from Atlantic bluefin tuna broodstocks maintained and induced to spawn in cages. An egg collection device will be used that includes a two-piece curtain deployed around the perimeter of the cage from 20 cm above to 3.5 m below the water level. Spawning induction will be undertaken during the peak of the spawning season. Eggs will be skimmed from the water surface every day at sunrise using fine mesh dip nets. The evaluation of the spawning quality will be carried out for all spawns.
Task 3.1 Description of the reproductive cycle of greater amberjack (led by UNIBA). Wild-caught broodstocks will be maintained in captivity by one of the SMEs. Broodstock will be sacrificed at three different times during the reproductive season. Blood, brains, pituitaries, gonads, muscle and liver will be sampled by scientist from different Partners to study the reproductive cycle, and spines will be collected for age determination. Wild fish will be sampled at the same time as a reference for evaluating reproductive function of captive fish. Proliferation and/or apoptosis of germ cells during spermatogenesis will be examined using Proliferating Cell Nuclear Antigen (PCNA) and the TUNEL method. Characterization of amberjack sperm will be done using CASA, looking at % spermatozoa motility, velocity, linearity of tracks, sperm membrane integrity by eosin/nigrosin, sperm viability and sperm energetic status using ATP measurement. A comparison of liver vitellogenin (Vg) and ovary Vg receptor (VgR) gene expression between captive and wild females will be assessed by cDNA sequencing and real time-PCR (qPCR); an analysis of oocyte yolk accumulation will be performed on histological sections using image analysis. Measurement of the sex steroid hormones T, E2, 11-KT and 17,20β DHP will be done using ELISAs and the expression of LH and FSH, as well as their plasma protein levels will be measured. Recombinant LH and FSH proteins will be produced and used to develop the respective hormone-specific ELISAs. Similar methodology will be applied to develop an ELISA for measuring leptin, a key metabolic hormone known to interact with the endocrine system to provide critical information about the nutritional status. The nutritional status of the captive and wild fish will be compared. The muscle, liver, and gonads samples will be analyzed to determine: (a) proximate and fatty acid composition, including specific lipid classes (i.e. phosphatidyl serine, inositol, choline and ethanolamine), (b) vitamin C and E and (c) carotenoids.
Task 3.2 Development of an optimized spawning induction protocol for captive greater amberjack in the Mediterranean (led by HCMR). Mature wild fish will be captured using a purse seine by two of the SMEs. Using these fish, captive broodstocks (n=10 males and females) will be established in HCMR, ARGO and ITICAL and will be maintained in tanks and cages. After 1 year in captivity (Y2), two different methods will be examined, either multiple GnRHa injections given every 7 days or implants for sustained release of GnRHa spanning 4-weeks. In Y3, two different doses of the most effective treatment from Y2 will be evaluated. In Y4, the timing of application (late June/early July) of the most effective treatment/dose will be optimized, based on the data obtained from the previous years. In the SME Partners, spawning induction on a single broodstock will be undertaken to validate protocols and to ensure egg production for the WP4 Larval husbandry. Evaluation of spawning quality will be carried out for all spawns, using individual egg incubations in 96-well microtiter plates.
Task 3.3 Development of an optimized spawning induction protocol for captive greater amberjack in the eastern Atlantic (led by FCPCT). Mature wild-caught fish adapted to culture conditions will be divided into 3 groups of 6 males and 6 females. At the onset of the spawning season in the eastern Atlantic (April-May), after evaluation of reproductive status fish will be treated with either single GnRHa injections every 7-10 days or GnRHa implants (that will be produced by HCMR) every 3 weeks, and one group will be left untreated as control. Evaluation of the spawning quality will be carried out for all spawns.
Task 3.4 Development of an optimized spawning induction protocol for F1 greater amberjack in the eastern Atlantic (led by IEO). Broodstock will be divided into 4 groups of 8 males and 8 females. At the beginning of the spawning season the reproductive status of fish will be evaluated (same procedures as 2.1.3; Mylonas et al., 2004) and fish treated with GnRHa implants. Females will be given GnRHa implants of different doses (0, 25, 50, 75 µg kg-1) while males will be implanted with 30 µg kg-1. The evaluation of the spawning quality will be carried out for all spawns (same procedure as 2.1.2; Mylonas et al., 2004). GnRHa implant treatments will be repeated when spawning stops. At the time of each GnRHa implantation and the end of the spawning season the following parameters will be determined: (a) plasma levels of the sex steroid hormones (HCMR); (b) plasma levels of triglycerides, cholesterol, protein and enzymes (GPT, GOT, alcaline phosphatase, cholinesterase and amylase); (c) cortisol, glucose and lactate; and (d) electrolytes, in order to determine hematological and biochemical indicators of health and welfare.
Task 3.5 Spawning induction of greater amberjack and egg collection in cages (led by HCMR). Cage spawning is expected to facilitate the management of greater amberjack broodstocks that may exceed 30 kg in commercial operations. Therefore, DIVERSIFY will employ methods used in a previous EU 7th FP project (SELFDOTT) for the collection of fertilized eggs from Atlantic bluefin tuna broodstocks maintained and induced to spawn in cages. An egg collection device will be used that includes a two-piece curtain deployed around the perimeter of the cage from 20 cm above to 3.5 m below the water level. Spawning induction will be undertaken during the peak of the spawning season. Eggs will be skimmed from the water surface every day at sunrise using fine mesh dip nets. The evaluation of the spawning quality will be carried out for all spawns.

How to train greater amberjack to feed on commercial extruded feeds
In nature, the greater amberjack Seriola dumerili feeds on fish and invertebrates (such as squid). In aquaculture, the existing broodstock come mostly from wild populations, and are fed either with live or frozen fish.
The objective of this protocol is to adapt broodstock from live/raw foods to semi-moist commercial extruded feeds. This is considered necessary because:
· Commercial feeds increase the biosecurity level since the risk of disease transmission from raw materials is eliminated,
· It is easier to manage fish feed, both financially (reduced cost of dry feed compared to live feed) and in terms of management (no need for freezer, etc.)
· The nutritional needs of broodstocks are covered to a greater extent with the commercial feeds, which include also special igredients for their reproductive function.
Diet with semi-moist (re-moisturized dry feed) was successfully used at the sea cage facility of the Hellenic Center for Marine Reserarch (HCMR) in Souda Bay, Crete, Greece, and then was applied in the Laboratory of Fish Reproduction, HCMR Aqualabs in Heraklion. The steps for implementing the above are:
1. Before the implementation of the protocol, fish had been fed with frozen mackerel three times per week (3% of their body weight),
2. The previous day of feeding, a quantity of dry food was placed (we used Vitalis Repro and Vitalis Cal, Skretting, Norway) in a plastic container, adding fresh water to a level of 1-2 cm below the surface of the feed pellets.
3. On feeding day, the moisturized dry feed was formed into balls (like meatballs), adding muscle of frozen fish (mackerel) in more than 80%, initially. The ball had a diameter of 2-2.5 cm.
4. If the fish did not accept these balls, we used the skin of mackerel’s slice (we had already removed the muscle) and filled up the created gap by a mixture of fish / moisturized dry feed, as in step 3.
5. At the beginning, we used offered a small quantity (1 ball / fish), even if the fish could eat more in order to continue to have appetite for the next day. After 7-15 days, when the fish adopted that way of feeding (and meanwhile gradually increased the amount to 2-3 balls / fish per day), we started to reduce the amount of muscle in the balls.
6. The decrease of the percentage of muscle could be decided by the person who feeds the fish. For example, if the fish accept easily feed with 50% of fish muscle, you could proceed to a reduction of the rate at the next feeding day (e.g. 30%).
7. About a month later, the fish accepted moisturized dry feed and they were fed with a quantity of 1% of their body weight (dry weight before adding water to the dry feed) three times a week, and then to 0.7% of their body weight (dry weight before adding water to the dry feed) five times a week (Monday to Friday).
8. Next step will be their training to dry feed in order to reduce significantly the labor involved (food preparation, food shaping into balls, etc..).
9. During the off-season we use Vitalis Repro. During the breeding season (March-July) we use Vitalis Cal, as more appropriate for the specific period. Other commercially available brood stock feeds are also appropriate.
In nature, the greater amberjack Seriola dumerili feeds on fish and invertebrates (such as squid). In aquaculture, the existing broodstock come mostly from wild populations, and are fed either with live or frozen fish.
The objective of this protocol is to adapt broodstock from live/raw foods to semi-moist commercial extruded feeds. This is considered necessary because:
· Commercial feeds increase the biosecurity level since the risk of disease transmission from raw materials is eliminated,
· It is easier to manage fish feed, both financially (reduced cost of dry feed compared to live feed) and in terms of management (no need for freezer, etc.)
· The nutritional needs of broodstocks are covered to a greater extent with the commercial feeds, which include also special igredients for their reproductive function.
Diet with semi-moist (re-moisturized dry feed) was successfully used at the sea cage facility of the Hellenic Center for Marine Reserarch (HCMR) in Souda Bay, Crete, Greece, and then was applied in the Laboratory of Fish Reproduction, HCMR Aqualabs in Heraklion. The steps for implementing the above are:
1. Before the implementation of the protocol, fish had been fed with frozen mackerel three times per week (3% of their body weight),
2. The previous day of feeding, a quantity of dry food was placed (we used Vitalis Repro and Vitalis Cal, Skretting, Norway) in a plastic container, adding fresh water to a level of 1-2 cm below the surface of the feed pellets.
3. On feeding day, the moisturized dry feed was formed into balls (like meatballs), adding muscle of frozen fish (mackerel) in more than 80%, initially. The ball had a diameter of 2-2.5 cm.
4. If the fish did not accept these balls, we used the skin of mackerel’s slice (we had already removed the muscle) and filled up the created gap by a mixture of fish / moisturized dry feed, as in step 3.
5. At the beginning, we used offered a small quantity (1 ball / fish), even if the fish could eat more in order to continue to have appetite for the next day. After 7-15 days, when the fish adopted that way of feeding (and meanwhile gradually increased the amount to 2-3 balls / fish per day), we started to reduce the amount of muscle in the balls.
6. The decrease of the percentage of muscle could be decided by the person who feeds the fish. For example, if the fish accept easily feed with 50% of fish muscle, you could proceed to a reduction of the rate at the next feeding day (e.g. 30%).
7. About a month later, the fish accepted moisturized dry feed and they were fed with a quantity of 1% of their body weight (dry weight before adding water to the dry feed) three times a week, and then to 0.7% of their body weight (dry weight before adding water to the dry feed) five times a week (Monday to Friday).
8. Next step will be their training to dry feed in order to reduce significantly the labor involved (food preparation, food shaping into balls, etc..).
9. During the off-season we use Vitalis Repro. During the breeding season (March-July) we use Vitalis Cal, as more appropriate for the specific period. Other commercially available brood stock feeds are also appropriate.