progress
 Figure 1: Capture Zone (NW Spain)
Figure 1: Capture Zone (NW Spain)
November 2014
Task 6.1 Collect wild fish to establish new broodstocks (led by CMRM, Fatima Linares).
Regarding this task, 3 wreckfish 2 fish with a fresh weight of 2 Kg each and 1 fish weighing 1.5 Kg, were captured using a “salabre” (Figure 1) since these fish are usually found below floating objects. The fishing area was located to 5 miles West of Corrubedo Cape, La Coruña.
Task 6.1 Collect wild fish to establish new broodstocks (led by CMRM, Fatima Linares).
Regarding this task, 3 wreckfish 2 fish with a fresh weight of 2 Kg each and 1 fish weighing 1.5 Kg, were captured using a “salabre” (Figure 1) since these fish are usually found below floating objects. The fishing area was located to 5 miles West of Corrubedo Cape, La Coruña.
Task 6.2 Describe reproductive cycle (led by IEO, Tito Peleteiro).
Blood samples were obtained from the CMRM (P19) stock (12 fishes) between January and June 2014 during the reproductive period in order to determine the evolution of sexual steroids (Figure 2). Samples from the ovaries were obtained and evaluated under the microscope to determine oocyte development stage.
A total of 60 fish were sampled between January and October of 2014 in the fish market (Figure 3) in order to obtain information of this species. Samples from the stomach, liver, gonads (Figure 4), muscle and fins were taken for biochemical (CMRM, ULL), genetic and cytology studies.
Blood samples were obtained from the CMRM (P19) stock (12 fishes) between January and June 2014 during the reproductive period in order to determine the evolution of sexual steroids (Figure 2). Samples from the ovaries were obtained and evaluated under the microscope to determine oocyte development stage.
A total of 60 fish were sampled between January and October of 2014 in the fish market (Figure 3) in order to obtain information of this species. Samples from the stomach, liver, gonads (Figure 4), muscle and fins were taken for biochemical (CMRM, ULL), genetic and cytology studies.
 Graphic 1: Total collected egg in Aquarium of Finisterrae
Graphic 1: Total collected egg in Aquarium of Finisterrae
Task
6.3 Development of spawning induction
procedures
(led by IEO, Tito Peleteiro).
A stock of 3 wreckfish (P1 HCMR), two males and a female Male and female was given a GnRHa implant. Two spawn was obtained, but a very small number of eggs were fertilized. Both fish were implanted again with GnRHa and were returned to the tank for spawning. Another spawn was obtained but again no fertilization was observed.
Sperm quality parameters were evaluated, after that was stored at 4°C for the following days, and was examined every other day for motility, until no forward motility was observed.
A stock of 12 wreckfish (P19 CMRM) weighing between 9.94 and 18.28 kg. This stock was sampled on a monthly basis to control sexual maturation and blood samples were collected to determine sexual steroids.
A stock of 9 wreckfish (P8 IEO). This stock was sampled twice a month during spawning season, in order to control sexual maturation. No evidence of sexual maturation was observed on females. Contrary, two males showed spermation.
A stock of 27 wreckfish (P32 MC2), weighing between 10.70 and 30.25 kg. Five females evident abdominal dilatation were isolated, as well as three fluent males. Ovary biopsies were made at 9 females, to determine oocytes stages. “In vitro” fertilization was performed, but spawn quality was poor, despite the oocytes were mature (2300µ in diameter).
The remaining two females spawned naturally in the tank. In almost all cases egg quality was poor, except the one from June 4th, with a fecundity percentage of 70% (Graphic 1). Nevertheless, this spawn was also of poor quality, since only 14% of these hatched.
A stock of 3 wreckfish (P1 HCMR), two males and a female Male and female was given a GnRHa implant. Two spawn was obtained, but a very small number of eggs were fertilized. Both fish were implanted again with GnRHa and were returned to the tank for spawning. Another spawn was obtained but again no fertilization was observed.
Sperm quality parameters were evaluated, after that was stored at 4°C for the following days, and was examined every other day for motility, until no forward motility was observed.
A stock of 12 wreckfish (P19 CMRM) weighing between 9.94 and 18.28 kg. This stock was sampled on a monthly basis to control sexual maturation and blood samples were collected to determine sexual steroids.
A stock of 9 wreckfish (P8 IEO). This stock was sampled twice a month during spawning season, in order to control sexual maturation. No evidence of sexual maturation was observed on females. Contrary, two males showed spermation.
A stock of 27 wreckfish (P32 MC2), weighing between 10.70 and 30.25 kg. Five females evident abdominal dilatation were isolated, as well as three fluent males. Ovary biopsies were made at 9 females, to determine oocytes stages. “In vitro” fertilization was performed, but spawn quality was poor, despite the oocytes were mature (2300µ in diameter).
The remaining two females spawned naturally in the tank. In almost all cases egg quality was poor, except the one from June 4th, with a fecundity percentage of 70% (Graphic 1). Nevertheless, this spawn was also of poor quality, since only 14% of these hatched.
 Graphic 2: Variations with time of Average Path Velocity i.e. along smoothed trajectory (dark grey) and Straight Line Velocity picturing the progressive movement (light grey)
Graphic 2: Variations with time of Average Path Velocity i.e. along smoothed trajectory (dark grey) and Straight Line Velocity picturing the progressive movement (light grey)
Task
6.4 Evaluation of sperm characteristics
and cryopreservation protocols (led by IFREMER, Christian Fauvel).
From April 8th to April 13th, sperm were collected from 6 males of la Coruña Aquarium , and 2 males from the facilities of the Instituto Español de Oceanografia (IEO) of Vigo all situated in Galicia (Spain). The mean concentration of wreckfish sperm in Galicia at this period was 2.41 1010 (sd :0.4 1010, n=9) spermatozoa per ml.
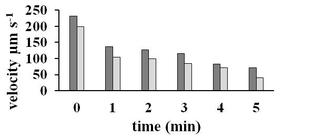
The two analyzed fresh sperm showed an initial velocity of more than 230µm per second which progressively decreased to 0 after 5 minutes (Graphic 2).
From April 8th to April 13th, sperm were collected from 6 males of la Coruña Aquarium , and 2 males from the facilities of the Instituto Español de Oceanografia (IEO) of Vigo all situated in Galicia (Spain). The mean concentration of wreckfish sperm in Galicia at this period was 2.41 1010 (sd :0.4 1010, n=9) spermatozoa per ml.
The two analyzed fresh sperm showed an initial velocity of more than 230µm per second which progressively decreased to 0 after 5 minutes (Graphic 2).
Participating organizations (WP leader in bold): P1. HCMR, P3. IRTA, P8. IEO, P14. IFREMER, P15. ULL, P19. CMRM AND P32. MC2
This WP includes tasks to (a) increase the availability of wreckfish broodstocks, (b) study the reproductive cycle of fish in captivity to understand the reproductive dysfunctions, (c) develop spawning induction methods for the acquisition of eggs and (d) develop methods for the study of wreckfish sperm and cryopreservation for in vitro fertilization applications.
Task 6.1 Collect wild fish to establish new broodstocks (led by CMRM). To increase the number of broodstock available wild fish, juveniles and adults will be collected from the wild. The collection of adult fish is complicated as these fish leave in great depths (>400 m) and all available broodstock were obtained from the wild as juveniles. The collected fish will be maintained under controlled conditions of temperature and photoperiod, and will be monitored every 4 months for growth and reproductive maturation, using gonadal biopsies. This task will both provide collection methods and availability of captive broodstock for SMEs and the aquaculture industry.
Task 6.2 Describe reproductive cycle (led by IEO). Due to the rarity of available wreckfish, in order to study its reproductive cycle in captivity, we will use all the available broodstocks in the EU, which are maintained in captivity at the facilities of HCMR (n=5), IEO (n=10), MC2 (n=24) and CMRM (n=12). Blood samples will be collected from the fish at different times during the annual reproductive cycle, and will be analyzed for the sex steroid hormones T, 11-KT, E2 and 17,20β-DHP using established ELISAs and gonadotropins (FSH/LH) using established non-homologous assays. Gonadal biopsies will be obtained at each sampling time and will be examined under the microscope to determine oocyte size and stage of development using wet mounts and histology. Collected sperm will be evaluated using standard sperm analysis (density, motility % and duration). In addition, wild fish from the fishery will be obtained during the reproductive period, and will be examined for gonadal development and nutritional status, in order to establish a base line in wild fish and compare with the data set obtained from captive broodstock.
Task 6.3 Development of spawning induction procedures (led by IEO). Available information from Partners of DIVERSIFY indicates that GnRHa implants (HCMR) may be effective in inducing oocyte maturation and ovulation, and that stripping protocols may be needed. Therefore, GnRHa implants will be used in the available stocks. All broodstocks will be monitored for reproductive function. When fish are at the correct maturation stage they will be induced to spawn using GnRHa implants, testing doses of 50-200 µg kg-1. Two approaches will be taken: (a) Fish will be placed in large tanks ≥40-m3 under controlled photothermal conditions and allowed to spawn spontaneously. Large tanks were shown in groupers to overcome the lack of spawning after ovulation. If fish fail to spawn, they will be sampled to identify failure of maturation or spawning, the latter being the experience in smaller tanks. Depending on the outcome, the approach will be adjusted with new doses (insufficient maturation) or strip spawning (lack of spawning). (b) Experiments will be conducted in smaller tanks (≤15 m3) and fish will be monitored for ovulation. Ovulated eggs will be inseminated in vitro using sperm from spermiating males and the eggs will be incubated. If fish fail to spawn, they will be sampled to confirm if the failure is related to lack of maturation and new implant doses will be tested. Eggs will be monitored for quality. Nutritional quality of egg batches will also be determined to compare with nutritional status of wild fish and to identify nutrients that may be lacking from the broodstock diet. Between the different stocks, husbandry variables such as sampling procedures, disturbance due to sampling, photothermal regime and nutrition (raw fish supplemented with commercial broodsotck diets) will be standardized and maintained as close to identical as possible. Standardization of these parameters will facilitate comparison of results from different stocks to determine optimal tank sizes, implant doses and spawning protocols.
Task 6.4 Evaluation of sperm characteristics and cryopreservation protocols (led by IFREMER). As indicated above, spawning induction methods for wreckfish should include in vitro fertilization, as spontaneous spawning is not achieved reliably (i.e., only in an Aquarium condition so far), and is expected to be a problem in industrial conditions. This dysfunction is common for other benthic and cave dwelling “groupers”, such as the dusky grouper (Epinephelus marginatus). Therefore, to enhance the operation of in vitro fertilization and dissociate sperm from egg collection, it is necessary to study sperm characteristics in wreckfish and develop cryopreservation methods. Characterization of wreckfish sperm will be done using CASA looking at % spermatozoa motility, velocity, linearity of tracks, sperm membrane integrity by eosin/nigrosin, sperm viability and sperm energetic status using ATP measurement. In order to increase the available volume, and perhaps the quality of the available sperm, GnRHa implants will be used to increase sperm availability, as has been achieved in other species. Males at the beginning of the spawning period (end of April) will be treated and sampled at 0, 3, 7 and 21 days afterwards. Sperm production (volume) and quality will be evaluated and the sex steroids T, 11-KT and 17,20β-DHP will be monitored in the blood to correlate with milt production. General cryopreservation protocols of marine fish sperm will be adapted to the specific requirements of wreckfish sperm. Furthermore, to test reliability of protocols, artificial insemination trials will be performed.
This WP includes tasks to (a) increase the availability of wreckfish broodstocks, (b) study the reproductive cycle of fish in captivity to understand the reproductive dysfunctions, (c) develop spawning induction methods for the acquisition of eggs and (d) develop methods for the study of wreckfish sperm and cryopreservation for in vitro fertilization applications.
Task 6.1 Collect wild fish to establish new broodstocks (led by CMRM). To increase the number of broodstock available wild fish, juveniles and adults will be collected from the wild. The collection of adult fish is complicated as these fish leave in great depths (>400 m) and all available broodstock were obtained from the wild as juveniles. The collected fish will be maintained under controlled conditions of temperature and photoperiod, and will be monitored every 4 months for growth and reproductive maturation, using gonadal biopsies. This task will both provide collection methods and availability of captive broodstock for SMEs and the aquaculture industry.
Task 6.2 Describe reproductive cycle (led by IEO). Due to the rarity of available wreckfish, in order to study its reproductive cycle in captivity, we will use all the available broodstocks in the EU, which are maintained in captivity at the facilities of HCMR (n=5), IEO (n=10), MC2 (n=24) and CMRM (n=12). Blood samples will be collected from the fish at different times during the annual reproductive cycle, and will be analyzed for the sex steroid hormones T, 11-KT, E2 and 17,20β-DHP using established ELISAs and gonadotropins (FSH/LH) using established non-homologous assays. Gonadal biopsies will be obtained at each sampling time and will be examined under the microscope to determine oocyte size and stage of development using wet mounts and histology. Collected sperm will be evaluated using standard sperm analysis (density, motility % and duration). In addition, wild fish from the fishery will be obtained during the reproductive period, and will be examined for gonadal development and nutritional status, in order to establish a base line in wild fish and compare with the data set obtained from captive broodstock.
Task 6.3 Development of spawning induction procedures (led by IEO). Available information from Partners of DIVERSIFY indicates that GnRHa implants (HCMR) may be effective in inducing oocyte maturation and ovulation, and that stripping protocols may be needed. Therefore, GnRHa implants will be used in the available stocks. All broodstocks will be monitored for reproductive function. When fish are at the correct maturation stage they will be induced to spawn using GnRHa implants, testing doses of 50-200 µg kg-1. Two approaches will be taken: (a) Fish will be placed in large tanks ≥40-m3 under controlled photothermal conditions and allowed to spawn spontaneously. Large tanks were shown in groupers to overcome the lack of spawning after ovulation. If fish fail to spawn, they will be sampled to identify failure of maturation or spawning, the latter being the experience in smaller tanks. Depending on the outcome, the approach will be adjusted with new doses (insufficient maturation) or strip spawning (lack of spawning). (b) Experiments will be conducted in smaller tanks (≤15 m3) and fish will be monitored for ovulation. Ovulated eggs will be inseminated in vitro using sperm from spermiating males and the eggs will be incubated. If fish fail to spawn, they will be sampled to confirm if the failure is related to lack of maturation and new implant doses will be tested. Eggs will be monitored for quality. Nutritional quality of egg batches will also be determined to compare with nutritional status of wild fish and to identify nutrients that may be lacking from the broodstock diet. Between the different stocks, husbandry variables such as sampling procedures, disturbance due to sampling, photothermal regime and nutrition (raw fish supplemented with commercial broodsotck diets) will be standardized and maintained as close to identical as possible. Standardization of these parameters will facilitate comparison of results from different stocks to determine optimal tank sizes, implant doses and spawning protocols.
Task 6.4 Evaluation of sperm characteristics and cryopreservation protocols (led by IFREMER). As indicated above, spawning induction methods for wreckfish should include in vitro fertilization, as spontaneous spawning is not achieved reliably (i.e., only in an Aquarium condition so far), and is expected to be a problem in industrial conditions. This dysfunction is common for other benthic and cave dwelling “groupers”, such as the dusky grouper (Epinephelus marginatus). Therefore, to enhance the operation of in vitro fertilization and dissociate sperm from egg collection, it is necessary to study sperm characteristics in wreckfish and develop cryopreservation methods. Characterization of wreckfish sperm will be done using CASA looking at % spermatozoa motility, velocity, linearity of tracks, sperm membrane integrity by eosin/nigrosin, sperm viability and sperm energetic status using ATP measurement. In order to increase the available volume, and perhaps the quality of the available sperm, GnRHa implants will be used to increase sperm availability, as has been achieved in other species. Males at the beginning of the spawning period (end of April) will be treated and sampled at 0, 3, 7 and 21 days afterwards. Sperm production (volume) and quality will be evaluated and the sex steroids T, 11-KT and 17,20β-DHP will be monitored in the blood to correlate with milt production. General cryopreservation protocols of marine fish sperm will be adapted to the specific requirements of wreckfish sperm. Furthermore, to test reliability of protocols, artificial insemination trials will be performed.