progress
November 2014
Task 18.1 Development of feeding methodology (lead by HCMR, Nikos Papandroulakis). This task was not accomplished, due to insufficient number of larvae available.
Task 18.2 Defining optimum conditions for larval rearing (lead by IEO, Tito Peleteiro).
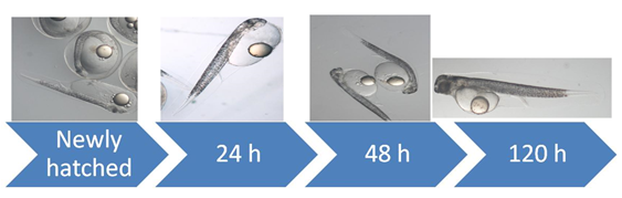
Sub-task 18.2.1. Larvae were only obtained spontaneously of abdominal massage from the Aquarium Finisterre stock (P. 32). Nevertheless, larval quality was very poor, compared to the previous year (Fig. 18.2.1), possibly due to extremely variable and changing weather conditions, which was the only changing variable. From all the spawns, only one had acceptable quality to attempt cultured.
Once egg selection was concluded (hatching percentage: 14%), the 11.340 larvae (3.8±0.3 mm) with 100% yolk sac were transferred to a 85 L culture tank. Yolk sac was consumed in 6 days after hatching (DAH); mortality was 100% at 20 DAH. Rotifers (Brachionus plicatilis), at a concentration of 8 rot/ml and phytoplankton were used as diet, but at no time ingestion of this live feed was observed. Larvae at 20 DAH had a functional digestive system but all presented empty stomachs.
Task 18.1 Development of feeding methodology (lead by HCMR, Nikos Papandroulakis). This task was not accomplished, due to insufficient number of larvae available.
Task 18.2 Defining optimum conditions for larval rearing (lead by IEO, Tito Peleteiro).
Sub-task 18.2.1. Larvae were only obtained spontaneously of abdominal massage from the Aquarium Finisterre stock (P. 32). Nevertheless, larval quality was very poor, compared to the previous year (Fig. 18.2.1), possibly due to extremely variable and changing weather conditions, which was the only changing variable. From all the spawns, only one had acceptable quality to attempt cultured.
Once egg selection was concluded (hatching percentage: 14%), the 11.340 larvae (3.8±0.3 mm) with 100% yolk sac were transferred to a 85 L culture tank. Yolk sac was consumed in 6 days after hatching (DAH); mortality was 100% at 20 DAH. Rotifers (Brachionus plicatilis), at a concentration of 8 rot/ml and phytoplankton were used as diet, but at no time ingestion of this live feed was observed. Larvae at 20 DAH had a functional digestive system but all presented empty stomachs.
Sub-task 18.2.2. This task was not accomplished, since biological material was not available, due to the poor larval quality from the MC2 (P 32) spawns.
Participating organizations (WP leader in bold): P1. HCMR, P8. IEO, P19. CMRM and P32. MC2
Task 18.1 Development of feeding methodology
Different feeding regimes (prey densities and succession of prey type) will be tested to develop a feeding protocol and avoid periods of food deprivation. Testing semi-intensive culture system (Mesocosm with 40,000 l) tanks, in triplicate trials from the end of endogenous feeding to the change to inert feeding (weaning phase). The culture system will be evaluated in terms of (1) ontogeny of larval digestive and visual system (influenced by feeding) through histological and image analysis procedures. In addition, the ontogeny of the digestive enzymes for wreckfish larvae will be studied under the different rearing regimes.
Task 18.2 Defining optimum conditions for larval rearing
Task 18.1 Development of feeding methodology
Different feeding regimes (prey densities and succession of prey type) will be tested to develop a feeding protocol and avoid periods of food deprivation. Testing semi-intensive culture system (Mesocosm with 40,000 l) tanks, in triplicate trials from the end of endogenous feeding to the change to inert feeding (weaning phase). The culture system will be evaluated in terms of (1) ontogeny of larval digestive and visual system (influenced by feeding) through histological and image analysis procedures. In addition, the ontogeny of the digestive enzymes for wreckfish larvae will be studied under the different rearing regimes.
Task 18.2 Defining optimum conditions for larval rearing
- Sub-task 18.2.1 Testing the effect of two temperature ranges (14-17 and 19-22°C) in triplicate trials in 2000 l tanks in flow-through systems and using the same photoperiod regime from the end of endogenous feeding to the change to inert feeding (weaning phase). These studies will be evaluated in terms of growth, survival, larval quality and size.
- Sub-task 18.2.2 Test of two culture systems RAS and flow-through in terms of larval culture conditions and feeding protocols. Trials will be evaluated in terms of biochemical profile (proteins, lipids and EFA content), and biometric analyses and survival.