Participating organizations (WP leader in bold): P3. IRTA and P15.ULL
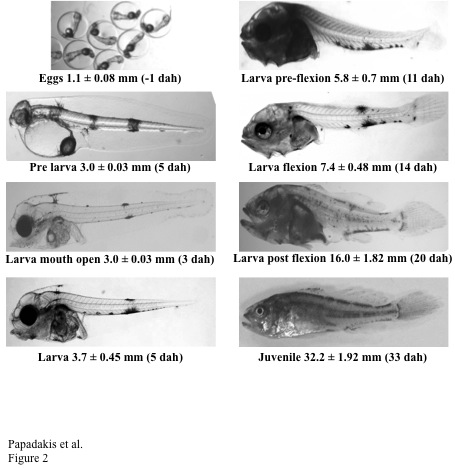
Larval rearing of meagre is relatively easy with a protocol generally based on the rotifer and Artemia feeding methodologies implemented in European sea bass and sea bream. However, meagre larvae are quite sensitive to high light intensity (more than 500 lux) or a long photoperiod. Although the precise requirements for essential amino and fatty acids are not completely known, the larvae show very good growth and survival rates using commercially available enrichment products for live prey. Meagre producers do not consider larval rearing to be a major stumbling block for meagre culture although cannibalism and variable size distribution in larvae and juveniles are the main concern as they would increase production costs and limit yields. Probiotic use at early stages of fish development may contribute to better face metamorphosis and weaning performance by stimulating growth, modulating digestive enzyme activity, improving intestinal epithelium integrity and altering biochemical composition. Therefore, advancing the early weaning of larvae from its dependence on Artemia on to a dry feed is a priority and the major focus of the larval work on meagre.
Task 14.1 Determining the earliest and most cost effective weaning period.
Larvae will be cultured intensively following a standard technique (rotifers from 2 days post-hatch-dph, Artemia nauplii from 12 dph until 30 dph) at low larval density and 12hL:12hD photoperiod (control group). In the experimental groups live prey supplemented or not with probiotics will be replaced at 7, 10 and 15 dph using commercially available weaning diets such as Gemma Micro (Skretting, Norway) or similar. Larval growth and size dispersion distribution, quality (typology and incidence of skeletal deformations), maturation of the digestive system in terms of activity of pancreatic and intestinal enzymes, as well as survival and larval biochemical composition will be analysed at the end of the experiment and compared to control larvae. The experiment will be carried out at least in triplicates (a 4x3 or 4x4 design) in 100 L tanks connected to a recirculation unit.
Larval rearing of meagre is relatively easy with a protocol generally based on the rotifer and Artemia feeding methodologies implemented in European sea bass and sea bream. However, meagre larvae are quite sensitive to high light intensity (more than 500 lux) or a long photoperiod. Although the precise requirements for essential amino and fatty acids are not completely known, the larvae show very good growth and survival rates using commercially available enrichment products for live prey. Meagre producers do not consider larval rearing to be a major stumbling block for meagre culture although cannibalism and variable size distribution in larvae and juveniles are the main concern as they would increase production costs and limit yields. Probiotic use at early stages of fish development may contribute to better face metamorphosis and weaning performance by stimulating growth, modulating digestive enzyme activity, improving intestinal epithelium integrity and altering biochemical composition. Therefore, advancing the early weaning of larvae from its dependence on Artemia on to a dry feed is a priority and the major focus of the larval work on meagre.
Task 14.1 Determining the earliest and most cost effective weaning period.
Larvae will be cultured intensively following a standard technique (rotifers from 2 days post-hatch-dph, Artemia nauplii from 12 dph until 30 dph) at low larval density and 12hL:12hD photoperiod (control group). In the experimental groups live prey supplemented or not with probiotics will be replaced at 7, 10 and 15 dph using commercially available weaning diets such as Gemma Micro (Skretting, Norway) or similar. Larval growth and size dispersion distribution, quality (typology and incidence of skeletal deformations), maturation of the digestive system in terms of activity of pancreatic and intestinal enzymes, as well as survival and larval biochemical composition will be analysed at the end of the experiment and compared to control larvae. The experiment will be carried out at least in triplicates (a 4x3 or 4x4 design) in 100 L tanks connected to a recirculation unit.